INTRODUCTION
In the European MCL Network Triangle study (Dreyling, ASH 2022) the addition of ibrutinib to frontline rituximab-containing chemotherapy and subsequent maintenance therapy improved failure-free survival in young, fit patients with mantle cell lymphoma (MCL). Ibrutinib was administered with R-CHOP, but not with R-DHAP, during the induction phase.
Continuous, uninterrupted Bruton Tyrosine Kinase (BTK) inhibition could maximize the benefit of front-line therapy given that responses develop over time. Acalabrutinib has less off-target BTK inhibition than ibrutinib. We hypothesize that combining continuous acalabrutinib with R-CHOP may result in a tolerable, outpatient, highly active regimen producing favorable outcomes.
METHODS
NCT04566887 is a phase II, single-arm, open-label study conducted across 5 academic centres in Canada. Patients ≥ 18 years of age with previously untreated MCL, ECOG performance status 0-2, adequate organ function and considered fit for autologous stem cell transplantation (ASCT) were included. Patients received 6 cycles of R-CHOP at standard doses plus acalabrutinib 100 mg twice per day orally. Responding patients proceeded with ASCT and maintenance rituximab and acalabrutinib for a total of 2 years.
The primary endpoint was the complete response (CR) rate after 6 cycles of induction with centrally reviewed PET/CT using the Lugano Criteria (Cheson, 2014). The total sample size was n=54 and the study completed accrual in May 2023. We present the results of the first 43 patients who have completed response assessment according to local investigators after 6 cycles of induction.
RESULTS
The median age was 62 years (range 38 - 69), 26 (60%) were male, 42 (98%) had performance status 0-1, 33 (78%) Ann Arbor stage IV, 35 (81%) bone marrow involvement, 6 (14%) high risk MIPI, 7 (16%) blastoid/pleomorphic morphology, 11 (26%) Ki67 ≥30%. All patients completed 6 cycles of induction and proceeded to ASCT. The overall response rate was 100%, and 39 (91%) patients achieved a CR. All patients started maintenance acalabrutinib + rituximab, although none have yet completed two years of protocol maintenance therapy.
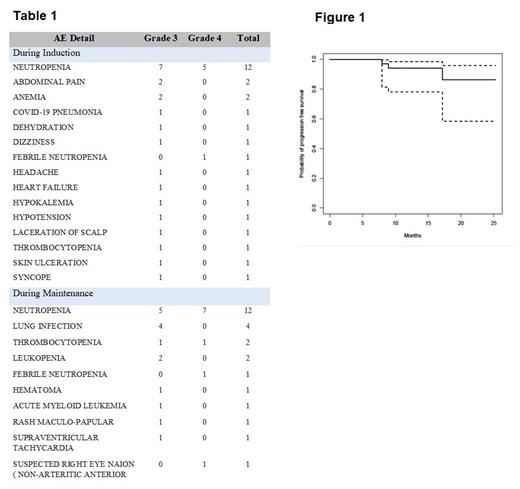
With a median follow up of 11.8 months (range 3.4 - 25.3) by the reverse Kaplan-Meier method, the 12-month PFS was 94% (95% CI 78.1 - 98.5), shown in Figure 1, and OS 95.5% (95% CI 71.9 - 99.3).
Three subjects have developed PD so far, all with adverse clinical and biological factors:
Baseline high-risk MIPI and Ki67 70%; biopsy at relapse TP53 positive by immunohistochemistry (IHC)
Baseline intermediate risk MIPI and Ki67 60%; biopsy at relapse with pleomorphic morphology, TP53 positive by IHC, Ki67 100%
Baseline high risk MIPI, Ki67 ≥30%, and blastoid morphology.
Table 1 lists common grade 3-4 adverse events (AE) during induction and maintenance. There were no grade 5 AE. Neutropenia was the most common AE during induction and maintenance. Most infections were respiratory. Cardiovascular toxicity was uncommon.
CONCLUSIONS
Acalabrutinib + R-CHOP is associated with a 91% CR rate with low rates of grade 3-4 AE expected for this combination. All patients have proceeded to ASCT and subsequent maintenance therapy. Maintenance therapy in this setting is associated with additional toxicity, particularly cytopenias and infections.
OffLabel Disclosure:
Villa:Roche, AstraZeneca, Abbvie, Janssen, Kite/Gilead, BMS/Celgene, BeiGene, Merck: Consultancy, Honoraria; Roche, AstraZeneca: Research Funding. Scott:Abbvie, AstraZeneca, Incyte: Consultancy; Janssen and Roche: Research Funding. Kuruvilla:Abbvie, Amgen, Astra Zeneca, BMS, Genmab, Gilead, Incyte, Janssen, Merck, Novartis, Pfizer, Roche, Seattle Genetics: Honoraria; Karyopharm: Other: DSMB; Abbvie, BMS, Gilead, Merck, Roche, Seattle Genetics: Consultancy; Roche, Astra Zeneca, Merck: Research Funding.
Frontline acalabrutinib in mantle cell lymphoma is currently considered off-label. The study has been approved by Health Canada.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal